FUNDING PORTFOLIO
The TANGO2 Research Foundation supports researchers and scientists at various stages of their careers in order to advance the understanding of TANGO2-deficiency disorder and basic disease mechanisms, identify innovative approaches to therapeutics, and develop strategies that support individuals living with TANGO2 deficiency disorder in carrying out their activities of daily living.
For half a decade, the TANGO2 Research Foundation has been successful in carrying out its mission and funded innovative basic and clinical research conducted by talented investigators worldwide. The TANGO2 Research Foundation has awarded close to $1,102,021 (USD) in funding since its inception, funding projects that have advanced the field and had a profound impact on children & young adults living with TANGO2 deficiency disorder.
Awarded Close To
1102021
Click a Research Cycle to View Corresponding Projects
- Current Research Projects (Grant cycle 5 / 2025-2026)
- Past Research Projects (Grant cycle 4 / 2024-2025)
- Past Research Projects (Grant cycle 3 / 2023-2024)
- Past Research Projects (Grant Cycle 2 / 2022-2023)
- Past Research Project (Open Cycle / 2022-2023)
- Past Research Projects (Grant Cycle 1 / 2020–2021)
- Other Research Projects Funded
RESEARCHERS

Dr. Lina Ghaloul-Gonzalez, MD
University of Pittsburgh Medical Center

Dr. Scott Lovell, PhD
University of Kansas
PROJECT
Novel Insights to TANGO2 Structure and Function
Abstract – TANGO2 deficiency disorder (TDD) is a rare autosomal recessive condition that presents with multi-systemic manifestations and phenotypic variability. While the precise function of TANGO2 remains unknown, studies suggest disruptions in ER/Golgi trafficking, mitochondrial bioenergetics, and lipid homeostasis as contributing factors to TDD pathology. Vitamin B5 (a substrate for coenzyme A; CoA) and folic acid have shown benefits in TDD. We recently solved the TANGO2 protein structure experimentally and identified a pocket, potentially interacting with key metabolites. Both CoA and 4’-phosphopantetheine binding to TANGO2 were modeled and both are positioned into the pocket to functionally interact with important amino acid residues. Additionally, our multi-omics data identified abnormalities in the folate/methionine cycles in TDD-derived fibroblasts when compared to control cells, pointing to abnormalities in both pyruvate dehydrogenase (PDH) and 10-formyltetrahydrofolate dehydrogenase (FTHFD) enzymes. Both enzymes depend on 4’-phosphopantetheinylation either directly or indirectly. FTHFD is linked to the folate/methionine pathway, making it particularly relevant given the demonstrated clinical effects of folate in TDD. PDH activation requires lipoylation, which in turn depends on lipoic acid synthesis—a process requiring 4’-phosphopantetheinylation of the mitochondrial acyl carrier protein (mtACP). Multiple metabolite findings indicate abnormal PDH function in TDD, including elevated pyruvate levels and a predicted decrease in acetyl-CoA. We hypothesize that TANGO2 is directly involved in the process of 4’-phosphopantetheinylation, facilitating the binding of 4’-phosphopantetheine to specific proteins through binding to CoA and hydrolyzing it to 4’-phosphopantethein. We further hypothesize that the metabolic disruptions and altered pathways observed in TDD are, at least in part, due to abnormal 4’-phosphopantetheinylation. This proposal’s aims are: (1) Investigate TANGO2 involvement in protein 4’-phosphopantetheinylation (4PPTylation) at a protein level; and (2) Examine the impact of TANGO2 on 4’-phosphopantetheinylation in cellular and metabolic function. With this study, we aspire to advance management strategies and enhance the quality of life for affected individuals.
Lay Summary – Significant progress has been made in recent years in understanding the pathophysiology of TANGO2 deficiency disorder (TDD), a disease that affects multiple organs and causes severe symptoms. Early studies have shown that vitamins B5 and folate may help reduce the severity of TDD symptoms, but how these vitamins help remains unclear. Our recent research suggests that the TANGO2 protein has a binding pocket, potentially interacting with an unknown metabolite. Using advanced multi-omics analyses, we’ve identified abnormalities in pathways linked to pyruvate and folate in TDD patient cells. Specific enzymes in these pathways require the addition of 4’-phosphopantethein to become active, a process connected to coenzyme A (CoA), which is derived from vitamin B5. We hypothesize that TANGO2 binds to and transports 4’-phosphopantetheine to activate these enzymes in the different pathways needed for normal cellular metabolism. Our aim is to understand TANGO2’s role in this process and uncover new insights into TANGO2’s function and identify biomarkers critical to developing targeted therapies.
Multi-Institution Award: $50,000
RESEARCHERS

Donna McDonald-McGinn. MS, LCGC
Children’s Hospital of Philadelphia

Dr. Beata Nowakowska, PhD
Institute of Mother and Child, Warsaw
PROJECT
Understanding TANGO2 Deficiency Disorder via Genotype-Phenotype Correlations in 22q11.2 Deletion Syndrome
Abstract – TANGO2 deficiency disorder (TDD) results from biallelic pathogenic variants in the TANGO2 gene. The 22q11.2 deletion syndrome (22q11.2DS) results in loss of ~50 genes, many considered important developmental drivers, including TANGO2, located within the LCR22A-LCR22B region on chromosome 22. Consequently, individuals with 22q11.2DS are at an increased risk of having TDD due to an additional variant on the non-deleted 22q11.2 allele. Given the potential severity of TDD and knowing that it is now treatable with Vitamin B Complex, it is imperative to diagnose everyone with 22q11.2DS and TDD. To date, we have identified TDD on a research basis in 3% of a sub-cohort of patients with 22q11.2DS followed at the Children’s Hospital of Philadelphia (CHOP) or via the Nowakowska Laboratory at the Institute of Mother and Child (Instytut Matki i Dziecka; IMID) in Warsaw, Poland. Thus, given the general population prevalence of 0.0001%, 22q11.2DS provides a unique opportunity to understand prevalence, genotype-phenotype correlations, and biomarkers for TDD. Here we propose to examine whole genome sequencing from two international 22q11.2DS consortia led by CHOP (IBBC and G2MH), and additional patients from Philadelphia and Warsaw, to detect pathogenic variants in TANGO2 in individuals with 22q11.2DS previously undiagnosed with TDD. Thereafter, we will confirm these variants using PCR and Sanger sequencing in the IMID lab, including all exons and intron/exon boundaries of the TANGO2 gene, followed by deep phenotyping by the CHOP team. Finally, all confirmed cases will be referred to the T2RF natural history study for long-term follow-up. This collaborative will elucidate biomarkers beyond those associated with 22q11.2DS alone, while providing additional insight into genotype-phenotype correlations for all patients with TDD. Moreover, this data will be key in providing genetic counseling in its entirety to families with 22q11.2DS and TDD and in supporting multidisciplinary care teams navigating the complexity of TDD management.
Lay Summary – 22q11.2 deletion syndrome (22q11.2DS) provides a unique and critical window into understanding TANGO2 Deficiency Disorder (TDD), as the deletion causes loss of ~50 genes on chromosome 22 including TANGO2, the most consequential gene within the region when there is no working copy on the non-deleted chromosome. Thus, people with 22q11.2DS are at great risk of also having TDD, compared with the general population where TDD requires changes in both copies of TANGO2. In fact, of 1668 patients with 22q11.2DS followed at the Children’s Hospital of Philadelphia (CHOP), we identified 5 with 22q11.2DS and a TANGO2 variant, including a 5-year-old child who suddenly succumbed prior to his diagnosis of TDD. Hence 3% of a subset of our cohort tested have TANGO2 variants. Additionally, 3 European patients were diagnosed with 22q11.2DS and TDD at the Institute of Mother and Child (IMID) in Warsaw. Consequently, since only a subset of patients with 22q11.2DS worldwide were tested for changes in TANGO2, as awareness of TDD is regrettably lacking, we propose to mine existing whole genome sequencing data generated by the NIH previously funded International 22q11.2 Brain and Behavior Consortium (IBBC) and Genes to Mental Health Network (G2MH), both international consortia led by Prof. McDonald-McGinn at CHOP, to identify patients with TDD and thereafter scrutinize the findings in these patients to establish correlations between their medical and cognitive/behavioral problems and their type of TANGO2 variants. Concurrently, we will examine changes in the TANGO2 gene in additional CHOP and IMID patients to provide genetic counseling in its entirety to families with 22q11.2DS and TDD and to generate data that will support the development of screening and healthcare guidelines for multidisciplinary care teams attempting to navigate the complexity of TDD management. Finally, we will refer all patients with 22q11.2DS and TDD to the T2RF Natural History Study.
Multi-Institutional Award: $50,000
RESEARCHERS

Georgia Sarquella-Brugada, MD
Institut de Recerca Sant Joan de Déu, Spain
PROJECT
Cardiac Characterization of Children with TDD: Understanding the Inter-Crisis Periods (IC-TDD)
Abstract – TANGO2 deficiency disorder (TDD) is a rare pediatric condition characterized by acute metabolic crises precipitated by infections, fasting, and high-fever episodes. During metabolic crises, most patients progress with muscle tissue breakdown (rhabdomyolysis) and cardiac crisis, which can lead to life-threatening ventricular arrhythmias and cardiomyopathy. Currently, antiarrhythmic events are the main cause of sudden cardiac death (SCD) in this population. In addition, although cardiac symptoms during crises are well documented, the cardiac physiology outside these episodes (inter-crisis periods) remains poorly understood.
The project aims are as follows: 1) to perform a comprehensive cardiac study in a pediatric cohort of TDD patients, using sequential echocardiography, electrocardiography, and continuous cardiac monitoring to investigate early subclinical cardiac impairment and the occurrence of arrhythmias (both during crisis and inter-crisis periods); and 2) to collect all information about TDD patients treated at our institution to be included in the TANGO2 Natural History Study and to position our hospital as a European reference satellite center for the care and promotion of TDD.
Our center monitors all TDD patients diagnosed in Spain to date, and our department performs a long-term evaluation of each TDD patient using implantable loop recorders (ILR) for continuous cardiac monitoring and detailed arrhythmia detection. By defining critical risk factors, we seek to identify early cardiac abnormalities, inform premature therapeutic interventions to alleviate cardiac symptoms, prevent life-threatening complications, and improve the quality of life of patients with TDD. Our proposal outlines a comprehensive and innovative approach to address critical knowledge gaps in the physiopathology changes prior to cardiac disease progression in a well-defined cohort TDD patients. This project seeks to define critical risk factors, inform early interventions, prevent complications, and improve the quality of life for TDD patients. This initiative underscores our commitment to pioneering research and providing exemplary care for TDD patients in Europe.
Lay Summary – TANGO2 deficiency disorder (TDD) is a rare inherited condition, that leads to severe metabolic crises often triggered by infections, fasting, or high fever episodes. During these crises, patients usually experience severe muscle breakdown (rhabdomyolysis) and heart problems, including dangerous irregular heartbeats (arrhythmias) and heart muscle damage (cardiomyopathy), with a high risk of sudden cardiac death (SCD). While some treatments help control heart issues during crises, they do not work for all patients. Additionally, physicians still do not fully understand how the heart is affected in between these critical episodes (a.k.a, inter-crisis periods).
To better understand how TDD affects the heart, we will closely monitor our patients using echocardiograms (pictures of the heart to see how well it’s working), electrocardiograms (ECG; tests to check the heart’s electrical activity and rhythm), and avant-garde implantable devices (small devices placed under the skin to monitor the heart activity remotely). We aim to detect heart problems before they become severe, so doctors can act quickly with treatments to prevent complications like SCD. To improve the detection, diagnosis and characterization of TANGO2 patients worldwide, we propose our institution as a European reference center for data collection of the TANGO2 Natural History Study led by the Baylor College of Medicine. By combining advanced cardiac monitoring with the enrichment of an international patient database, this project will improve our understanding of how TDD affects the heart, help apply better treatments to reduce the risk of SCD, and ultimately provide exemplary care for TDD patients, thereby improving their quality of life.
Single Institutional Award: $25,000
RESEARCHERS

Andreas Roos, PhD
University Medicine Essen; Duisburg-Essen Univ.

Bert Klebl, PhD
University Medicine Essen; Duisburg-Essen Univ.
PROJECT
TT2: Treat TANGO2 Deficiency(IC-TDD)
Abstract – The purpose of this research project, TT2 (Treat TANGO2-deficiency), is to develop targeted therapeutic interventions for TANGO2-deficiency, a rare and currently untreatable neuropediatric disorder characterized by recurrent metabolic crises, hypoglycemia, lactic acidosis, rhabdomyolysis, arrhythmias, and cognitive decline. The specific goals of TT2 are to unravel the complex pathomechanisms of TANGO2-deficiency and to discover potential drug candidates that can restore cellular function, thereby improving clinical outcomes for affected patients.
The research design will employ a multi-omics approach using patient-derived fibroblasts, which will be analyzed through transcriptomic, proteomic, metabolomic, and lipidomic profiling. These fibroblasts, obtained minimal-invasively from skin biopsies, will serve as a model for the disease, circumventing the need for more invasive procedures like muscle or nerve biopsies. The integrated omics data will be analyzed using artificial intelligence (AI) to identify key molecular pathways involved in disease progression. Based on these findings, AI will also be used to predict existing drugs that could target these pathways, with the most promising candidates selected for further testing.
The methods will involve pre-clinical testing of the identified drugs in patient-derived fibroblasts. Cellular assays, including assessments of cellular viability, autophagic flux, and proteasomal activity, will be performed to evaluate the efficacy of the drugs in restoring cellular homeostasis. These assays will complement the multi-omics data to ensure a comprehensive evaluation of the drugs’ effects.
This research is significant because it will provide critical insights into the pathophysiology of TANGO2-deficiency and enable the identification of potential treatments through a “bench-to-bedside” approach. By using patient-derived fibroblasts and AI-driven drug repurposing, TT2 will offer a rapid, cost-effective strategy for finding therapies for TANGO2-deficiency. Consequently, the results of this study will inform individualized treatment strategies for patients, improving the prospects for clinical management and advancing the field of rare disease research.
Lay Summary -TANGO2-deficiency is a rare genetic disorder that affects the brain, muscles, and other organs, causing serious health issues such as metabolic crises, low blood sugar, muscle breakdown, heart problems, and cognitive decline. Currently, there is no cure for the condition, and treatment options are limited. This research project, TT2, aims to identify the causes of TANGO2-deficiency and find potential treatments by studying cells from patients with the condition.
To do this, we will use skin cells, called fibroblasts, which can be easily obtained without the need for invasive procedures. We will analyze these cells using advanced technologies that look at genes, proteins, and other molecules inside the cells to understand what goes wrong in TANGO2-deficiency. Artificial intelligence (AI) will help us identify key molecular pathways involved in the disease and predict existing drugs that might help treat the condition. The most promising drug candidates will then be tested in the laboratory on these patient-derived cells.
In addition to testing the drugs, we will assess how well they help restore the cells’ normal functions. This research is important because it will provide new insights into how TANGO2 deficiency works and help identify potential treatments that can be quickly tested and used for patients. By using patient-specific cells and AI, TT2 offers a faster, more personalized approach to finding treatments for rare diseases. The ultimate goal is to provide individualized care for TANGO2 patients and improve their quality of life.
Single Institution Award: $18,500
RESEARCHERS

Samuel Mackenzie, MD, PhD
(PI)
University of Rochester

Vandana A. Gupta, PhD
(PI)
Brigham and Women’s Hospital
PROJECT
Developing Mechanism-based therapies for TDD
Abstract – TANGO2 deficiency disorder (TDD) is an early-onset disorder caused by recessive mutations in the TANGO2 gene. Although promising therapies are being developed, and B-complex vitamins seem to mitigate many of the more severe features of TDD, there is no cure. Even though the disease is monogenetically defined, there remains great variability in disease onset and progression across patients,likely due tog enetic and environmental factors. TANGO2 has been indirectly implicated in multiple biological processes, from cellular trafficking to maintaining mitochondrial structure and function. Extrinsic factors contributing to disease onset and/or exacerbating the disease pathology in TDD have recently been shown to target some of these processes regulated byTANGO2. However, detailed functional roles of TANGO2 indifferent tissues and which specific defects can be therapeutically targeted to restore normal states have yet to be discovered. By taking a multi-organism and mutation-independent approach, our central aim is to develop effective therapeutics for patients affected by TDD. Leveraging the expertise of our labs working with different model systems of TDD(nematode and zebrafish), we will conduct a small molecules-based screen of two libraries containing (a) FDA-approved compounds targeting diverse biological processes and (b) mitochondrial targeting compounds controlling different mitochondrial-regulated pathways.These studies will identify effective therapeutic compounds and provide novel insights into the pathophysiology ofTDD.
Lay Summary – TANGO2 deficiency disorder (TDD) is a multisystemic disorder affecting brain, skeletal muscle, and heart function. Life-threatening symptoms in patients are provoked by exposure to external triggers such as viral illness or fasting. While recent data suggest that selective vitamins may help alleviate some symptoms of TDD, the mechanism by which vitamins help is not known, nor is their effect thought to be fully curative. To accelerate the discovery of more potent therapies for individuals with TDD, we propose to use TANGO2 nematode and zebrafish models to screen thousands of possible compounds. By determining which compounds rescue key disease features in nematodes and zebrafish, we can identify a much smaller list of compounds to test in a more in-depth fashion. This work will accelerate the identification of promising therapeutic candidates as we move toward work in other species and clinical trials.
Multi-Institution Award: $50,000
RESEARCHERS

Lina Ghaloul – Gonzalez, MD
(PI)
UPMC

Barbara Bakker, prof, dr, BM
(Co-I)
University of Groningen

Ody Sibon, PhD
(Co-I)
University of Groningen
PROJECT
Investigating Co-A imbalance, vitamin B5’s role, and metabolomics in TANGO2 Deficiency Disorder
Abstract – TANGO2 deficiency disorder (TDD) is a rare autosomal recessive disorder affecting approximately 8000 people worldwide, presenting with multisystemic manifestations and phenotypic variability. While the precise function of TANGO2 remains unknown, studies suggest disruptions in endoplasmic reticulum (ER)/Golgi trafficking and mitochondrial dysfunction as contributing factors to the pathology of TDD. Though no cure exists, vitamin B5 supplementation shows promise in alleviating symptoms partially. Our proposed research aims to elucidate the underlying mechanisms of B5’s therapeutic effects in TDD, with a focus on its role as a precursor for coenzyme A (CoA). CoA is highly concentrated in the mitochondria and plays a crucial role in metabolism including fatty acid synthesis, fatty-acid β-oxidation, the Krebs cycle, and protein posttranslational modifications, including acetylation. Utilizing Tango2 mutant Drosophila melanogaster and patient-derived fibroblasts, we will investigate the potential imbalance of CoA in TDD and the efficacy of CoAprecursors in correcting TDD phenotypes. Additionally, we will conduct untargeted metabolomics studies to uncover novel pathways involved in TANGO2 function and identify potential interactions between TANGO2 protein and metabolites. These comprehensive investigations aim to enhance our understanding of the therapeutic mechanisms of B5 and thereby maybe identify strategies to enhance its positive effect. In addition, our research may reveal alternative therapeutic avenues for TDD. The proposed research has two specific aims, namely (1) investigation of a possible imbalance of CoA in TDD and the restorative role of CoAprecursors in correcting TDD; and (2) untargeted metabolomics and identification of TANGO2 protein-metabolite interactions. Ultimately, this study’s findings will guide the development of targeted interventions, improving the well-being of those affected. By unraveling the complex interactions among B5, CoA, and metabolic pathways—particularly those in mitochondria such as fatty-acid β-oxidation and the Krebs cycle—we aspire to advance management strategies and enhance the quality of life for affected individuals.
Lay Summary – In recent years, we’ve made significant progress in understanding TANGO2-related disorder (TDD), a condition that affects multiple organ systems. We’ve also observed the positive impact of vitamin B5 in reducing the severity of the disease. However, there’s still a gap in our knowledge regarding how this improvement occurs. This knowledge is important and will provide clues on how to further enhance the improvement by vitamin B5. We know that B5 is a building block for coenzyme A (CoA), a crucial molecule in cell metabolism. Our hypothesis is that TDD patients may have a shortage of CoA, and by providing them with vitamin B5, we can increase their CoA levels. This boost in CoA enhances cell metabolism and leads to an improvement in clinical symptoms. To test this theory, we will measure the level of CoA in TDD cells and fruit flies affected with TDD. This will help us determine if there is indeed a CoA deficiency or if certain CoA metabolites are in abundance while others are depleted. Furthermore, we will examine the levels of various molecules (metabolites) within the TDD cells that may be abnormal and study the impact of vitamin B5, if any, on these metabolites. This comprehensive approach will shed light on which pathways are most affected by TANGO2 and guide us in identifying the most promising therapeutic options for treating TDD.
Multi-Institution Award: $50,000
RESEARCHERS

Vandana A. Gupta, PhD
(PI)
PROJECT
Metabolic and Genetic Modifiers of TANGO2-deficiency Disorder
Abstract – Recessive mutations in TANGO2 underlie a rare pediatric disorder resulting in encephalopathy, rhabdomyolysis, and cardiac abnormalities. Despite the knowledge of the genetic defect, the cause of extreme clinical heterogeneity and specific triggers that contribute to clinical complications remains mostly unknown, and no specific treatments exist for these patients. In Aim 1, we will develop a comprehensive map of pathological defects during disease onset and progression in different organs that result in lethality in Tango2 deficiency at the base level and in the presence of metabolic stress. Identifying modifier genes and pathways that underlie a mild phenotype in a small group of tango2 mutants in Aim 2 will provide targets for disease therapy and drug discovery. Success in this work will lead to a better understanding of the cause of clinical heterogeneity and the development of new therapeutic targets in TANGO2 patients.
Lay Summary – The TANGO2-associated disorder is a complex and heterogeneous disease. Events of clinical episodes in TANGO2 patients are provoked by exposure to external triggers in combination with increased genetic susceptibility, which affects the daily quality of life and can lead to life-threatening complications. The need for suitable animal models has hindered disease understanding and preclinical therapeutic developments. To address these critical issues, we have developed a zebrafish model of Tango2 deficiency that recapitulates the clinical spectrum of phenotypes in human patients. Therefore, it provides a robust system for investigating disease processes in cardiac and skeletal muscle and nervous systems and treatment approaches. Completion of these studies will generate basic and clinical insights on the key regulators of disease onset and progression and will be used as a guide for the advancement of therapeutic approaches and suggest avenues for the prevention of health-to-disease transition.
Single Institution Award: $25,000
RESEARCHERS

Lilei Zhang, MD, PhD
(PI)

Aleksandar Bajic, PhD
(Co-I)
PROJECT
Understanding neurodevelopment in TANGO2 deficient disease
Abstract – The neurodevelopmental symptoms have the most significant impact on the quality of life of TANGO2 deficient patients and their families. Unfortunately, there has been no study so far directly addressing the pathophysiology of the neurodevelopmental disease. We took advantage of our established iPSC and isogenic controls and combined them with KO mouse model to investigate the cellular and molecular mechanism of TANGO2 deficiency in human neuronal cell types and in vivo models. In evaluating the neurobehavior phenotype of TANGO2 KO mice, we will in particular be focusing on the impact of vitamin B, which will provide direct insights to therapy.
Lay Summary – While TANGO2 deficient disease (TDD) is a multi-system disease, the neurodevelopmental symptoms are most important for the life quality of the patients and their family. There has been no study so far directly addressing the pathophysiology of the neurodevelopmental disease. We have previously generated patient specific induced pluripotent stem cells and their matching control on the same genetic background. Using these cell pairs, we were able to generate heart cells and characterize the mechanism and treatment for TANGO2 associated arrhythmias. We may differentiate the same stem cells into neuronal cells to understand the effect of TANGO2 deficiency in the brain. Further, we have in hand TANGO2 deficient mouse models, which was able to recapitulate cardiac arrhythmias, but neurobehavior studies have not been done. In this proposal, we will use both iPSC-neuronal cell model and the mouse in vivo model to understand the molecular mechanism of TANGO2 deficiency in the brain, which will help us design specific treatment strategies.
Multi-Institutional Award: $35,000
RESEARCHERS

Michael Sacher, PhD
(PI)

Chiara Gamberi, PhD
(Co-I)
PROJECT
A lipidomic analysis of multiple model systems to better understand the role of TANGO2 in lipid homeostasis
Abstract – Accumulating evidence from us and others suggests that TANGO2 plays an important role in ensuring lipid balance (homeostasis) within the cell. Such a hypothesis is consistent with previous studies that suggested defective lipid metabolism as the most likely explanation for the phenotypes seen in individuals affected by TANGO2 deficiency disease (TDD). Supportive evidence includes a study that demonstrated lipid imbalance upon depletion of TANGO2 and our recent publication showing that vitamin B5, a precursor to the lipid species called acyl-Coenzyme A (acyl-CoA) rescues key TANGO2 loss-of-function phenotypes. Moreover, our unpublished finding suggests an interaction between the TANGO2 protein and an enzyme that regulates acyl-CoA levels as well as our preliminary finding of profound changes in the lipid profile in fibroblast cells from an individual harboring a TANGO2 mutation. The Sacher and collaborating Gamberi laboratories have been studying the function of TANGO2 in three different model systems including human cells, fruit flies and yeast cells. Here, we propose to identify which lipid species are altered and which lipid pathways are dysregulated in the absence of functional TANGO2 employing combined molecular and genetic analyses in three model systems. Although several lipid species have been found to be altered in human cells devoid of TANGO2, a comparative analysis in the three model systems will facilitate identification of the key lipid(s) and pathways involved in TDD. Several hypotheses as to how TANGO2 regulates lipid homeostasis will be tested biochemically in vitro with purified components. We are confident that the proposed integrative research will make important strides in closing in on the role of TANGO2 in lipid homeostasis, a function likely to be dysregulated in the diseased state. Our study will also shed light on how vitamin B5 affects the lipid profile and may suggest alternative or additional treatment options.
Lay Summary – Accumulating evidence from us and others suggests that TANGO2 plays an important role in ensuring lipid balance (homeostasis) within the cell. Such a hypothesis is consistent with previous studies that suggested defective lipid metabolism as the most likely explanation for the phenotypes seen in individuals affected by TANGO2 deficiency disease (TDD). Supportive evidence includes a study that demonstrated lipid imbalance upon depletion of TANGO2 and our recent publication showing that vitamin B5, a precursor to the lipid species called acyl-Coenzyme A (acyl-CoA) rescues key TANGO2 loss-of-function phenotypes. Moreover, our unpublished finding suggests an interaction between the TANGO2 protein and an enzyme that regulates acyl-CoA levels, ACOT1, as well as our preliminary finding of profound changes in the lipid profile in fibroblast cells from an individual harboring a TANGO2 mutation. The Sacher and collaborating Gamberi laboratories have been studying the function of TANGO2 in three different model systems including human cells, fruit flies and yeast cells. Here, we propose to identify which lipid species are altered and which lipid pathways are dysregulated in the absence of functional TANGO2 employing combined molecular and genetic analyses in three model systems. Although several lipid species have been found to be altered in human cells devoid of TANGO2, a comparative analysis in the three model systems will facilitate identification of the key lipid(s) and pathways involved in TDD. Several hypotheses as to how TANGO2 regulates lipid homeostasis will be tested biochemically in vitro with purified components. We are confident that the proposed integrative research will make important strides in closing in on the role of TANGO2 in lipid homeostasis, a function likely to be dysregulated in the diseased state. Our study will also shed light on how vitamin B5 affects the lipid profile and may suggest alternative or additional treatment options.
Multi-Institutional Award: $50,000
RESEARCHERS

Pr Alfredo Brusco
(Co-PI)

Pr Giovanni Ferrero
(Co-PI)
PROJECT
Understanding TANGO2 pathogenic mechanisms in hiPSCs-derived neurons
Abstract – TANGO2 gene is associated with an autosomal recessive disorder characterized by recurrent metabolic encephalomyopathic crises with rhabdomyolysis, cardiac arrhythmias, and neurodegeneration (MIM #616878). Despite the scientific efforts, pathogenic mechanisms underlying TANGO2-related disease are still largely unknown, as well as the reasons behind the pleiotropic nature of the syndrome and the significant clinical variability among patients.
Because an important lack of knowledge relates to the impairment of the neurological compartment, our work will focus on investigating the role of TANGO2 in neurons and provide initial insights into the genetic bases of the disease clinical variability.
We will generate human induced pluripotent stem cells (hiPSC) from fibroblasts of two siblings with the same TANGO2 genotype, but clinically discordant (one affected by a severe, the other by a mild/inapparent form). Patients-derived hiPSC will be used to obtain neuronal progenitor cells (NPC) and mature neurons in vitro. Using these cells, we will study cellular morphology and vitality, and perform a transcriptome analysis, comparing the two siblings with healthy control subjects. In addition, we will perform exome sequencing of patients’ DNA, to better investigate the potential impact of variants in genes for TANGO2-related proteins. With our project, we will combine next generation sequencing technologies (exome sequencing and transcriptome) and innovative in vitro models (hiPSCs-derived NPC and neurons), to shed light on altered pathways in neurons and the mechanism behind the different severity degrees observed in patients, potentially identifying genetic modifiers of the disease.
Lay Summary – This work will provide new insights into the pathogenesis of TANGO2 disease. We will develop in vitro tools to study the disease, namely hiPSC and hiPSC-derived neuronal progenitor cells (NPC) and mature neurons from two clinically discordant TANGO2-affected siblings. Using morphologic and transcriptome analyses, we will provide insights into the role of TANGO2 in the brain, improving the knowledge on the network of TANGO2 gene interactions and pathways. Finally, we will obtain data to start understanding variable expressivity of this disease.
Single Institution Award: $25,000
RESEARCHERS

Lina Ghaloul-Gonzalez, MD
(PI)
PROJECT
Investigating the effect of mitochondrial stabilizing compounds on TANGO2 deficient cells
Abstract – TANGO2 deficiency is an autosomal recessive disorder caused by mutations in the TANGO2 gene. Under stress/illness patients with TANGO2 deficiency can develop acute metabolic crisis, rhabdomyolysis and/or cardiac arrhythmias. Genotype/phenotype correlations have been poor with significant inter and intra-familial variation, suggestive of the existence of gene modifiers leading to diversity in clinical presentation. There is not much known about the pathophysiology of the disease. Functional studies including ours revealed that TANGO2 protein is present in mitochondrial extracts of control cells. Additionally, we were able to demonstrate that patient derived TANGO2 deficient cells have dysfunction of mitochondrial energy metabolism. However, we were unable to do any immunofluorescence staining studies (IF) for subcellular localization of TANGO2 due to the lack of specific TANGO2 antibodies that are suitable for use for IF studies. I hypothesize that TANGO2 is present in different subcellular compartments and plays a role in protein transportation/trafficking between organelles. In addition, I hypothesize that disruption of TANGO2 in patients with missense variants leads to toxic accumulation of it in one or more cellular compartments. To test this hypothesis, we need to generate specific TANGO2 antibodies that are suitable to be used for IF studies. We have started the process of purifying TANGO2 protein to be used for antibody production. In addition, due to the previous studies including ours showing mitochondrial dysfunction, I will be studying the effect of mitochondrial stabilizing compounds on mitochondrial function in patient cells.
Lay Summary – How defects in the TANGO2 gene cause disease is unknown. Our previous studies have shown that patient cells have an impaired ability to make energy, possibly related to transport of critical proteins into mitochondria, the powerhouse of cells. Studies on TANGO2 protein have been hindered by the lack of a good antibody that recognizes the protein in lab experiments. In this project, I propose to generate TANGO2 antibodies for lab studies. I will also test possible drugs to treat TANGO2 deficiency in cell studies as a necessary first step for studies in patients.
Single Institution Award: $25,000
RESEARCHERS

Samuel Mackenzie, MD, PhD
(PI)
PROJECT
Remote Assessment of Abnormal Movements in TANGO2 Deficiency Syndrome
Abstract – Fundamental questions remain surrounding the clinical manifestations of TANGO2 deficiency syndrome (TANGO2- DS). There are multiple accounts of children experiencing episodes of fatigue, weakness, and fussiness early in life that were not accurately classified at presentation, leading to delayed diagnosis of the condition and, by extension, a lack of appropriate treatment. Retrospective case series of patients with TANGO2-DS reveal that about 50% of patients experience movement disorders (ataxia, dystonia, etc.) at some point in their lives. The true prevalence is likely higher due to underreporting and underdiagnosis. As no study to date has specifically examined movement disorders in TANGO2-DS, this project aims to improve collective understanding of the range of abnormal movements and other behavioral episodes observed in this condition by remote video assessment. To accomplish this, families will submit videos to us via a secure platform, and a team of pediatric movement disorder neurologists will review these videos and characterize the events by phenomenology. Finally, we will explore how different movement phenomenologies may correlate with other disease characteristics (e.g., types of variants in the TANGO2 gene, context of situation in which event occurred, alleviating/exacerbating symptoms). Information about additional characteristics will be collected by online surveys and/or phone interviews with parents and care providers. This work has the potential to improve TANGO2-DS diagnosis rates, inform our pathophysiological understanding of the disease, and contribute to clinical trial readiness as we move toward potential treatments for TANGO2-DS in the not-so-distant future.
Lay Summary – Abnormal movements experienced by children with TANGO2-deficiency syndrome (TANGO2-DS) are known to occur fairly commonly, though to date, no group has comprehensively characterized them. Based on published and anecdotal reports, such movements might include episodes of imbalance, wobbly gait, poor coordination, tilting to one side, or limb stiffness. In many cases, the medical team is unable to provide a clear diagnosis for these events, leaving families with uncertainty about how to manage symptoms. Accurately identifying these abnormal movements may be the first step towards making a diagnosis of TANGO2-DS or creating a treatment plan. It may also provide clues as to what is happening in the disease. In this study, a panel of pediatric movement disorder specialists will review videos of children with TANGO2-DS. They will then decide what to call each of the movements in medical terms. Additionally, they will use information obtained from surveys to determine how other features (e.g., abnormalities in the TANGO2 gene, situation in which an event occurred, and factors that make symptoms better or worse) affect why or when these movement events occur.
Single Institution Award: $12,500
RESEARCHERS

Michael Sacher, PhD
(PI)

Chiara Gamberi, PhD
(Co-PI)
PROJECT
Exploring a novel TANGO2 interacting partner as a new
mechanism for the role of TANGO2 disease
Abstract – Mutations in TANGO2 result in illness- or diet-induced rhabdomyolysis, life threatening cardiac arrhythmias, lactic acidosis and metabolic crises. While TANGO2 was originally suggested to function in membrane trafficking, we and others have shown it localizes to mitochondria. More recently, we have identified a novel interacting partner of TANGO2. This putative interactor is involved in regulating acyl-CoA levels in a cell. Furthermore, fruit flies with a TANGO2 mutation show reduced mobility and reduced survival upon starvation which is partially rescued by vitamin B5, a precursor of CoA. In this proposal we will focus on the physiological relevance of this novel interactor and its consequence on fatty acid/energy metabolism. We will examine if stress increases the interaction and if dysfunctional TANGO2 affects the interacting protein activity upon stress. The interaction will be examined in both mouse and human myotubes as a muscle model system, utilizing either a TANGO2 knockout or naturally occurring human TANGO2 pathological variants. We will also examine the interaction in a reconstituted in vitro system with purified components. The fruit fly model system will be used to probe how TANGO2 affects CoA and lipid metabolism. We will also generate flies expressing human TANGO2 to study genetic and molecular interactions in vivo and establish a model for testing hypotheses derived from the mammalian and in vitro studies and efficiently test pharmacological interventions. Together, this will provide a powerful system to address functional consequences of the first TANGO2 interactor identified to date.
Lay Summary – We have discovered a novel interacting partner of TANGO2. The putative partner influences the pool of fatty acids that can be utilized by the cell to produce energy. We suspect that TANGO2 negatively regulates this protein to allow for persistence of the pool of available fatty acids. Thus, individuals with TANGO2 mutations will suffer from an energy imbalance. Our studies will explore this hypothesis using human and mouse muscle cells, in vitro biochemical studies as well as fruit fly genetics and physiological assays.
Multi-Institutional Award: $50,000
RESEARCHERS

Samuel Mackenzie, MD, PhD
(PI)

Lili Wang, PhD
(Co-PI)
PROJECT
Investigating the role of reactive oxygen species in TANGO2 deficiency syndrome
Abstract – TANGO2 deficiency syndrome (TANGO2-DS) is a phenotypically heterogeneous, autosomal recessive condition characterized by episodic rhabdomyolysis, metabolic crises, developmental delay, seizures, movement disorders, and cardiac arrhythmias. Many researchers have speculated that TANGO2-DS shares a common pathophysiological basis with fatty acid oxidation disorders given the phenotypic overlap observed in patients; however, the function and localization of the TANGO2 protein remains poorly understood. In this study, we will investigate the possibility that TANGO2 is implicated in the production or processing reactive oxygen species (ROS), small molecules that are known to impair bioenergetic function at high concentrations. Using healthy myotubes and cardiomyocytes, we will determine what other proteins TANGO2 may interact with to perform its role in mitigating ROS-mediated pathology. In TANGO2-deficient cells, we will also determine the effect of different substrate conditions on oxygen consumption rate, energy production, levels of ROS, contractility, and viability. If ROS are implicated as the basis for TANGO2-DS as a result of this work, our findings will prompt additional studies employing targeted therapeutics (e.g., ROS scavengers) with the aim of lessening or rescuing the disease phenotype.
Lay Summary – The clinical and laboratory features of TANGO2 deficiency syndrome (TANGO2-DS) suggest that the breakdown of fats in cells may be impaired in some way. This study will use human cells to investigate whether reactive oxygen species (ROS), which are generated when energy is produced in mitochondria and are known to cause cell damage at high levels, are relatively increased in TANGO2-DS when cells are forced to use various forms of fat as fuel. An absence of TANGO2 protein may result in more ROS being produced or less ROS being cleared, and we will explore both of these possibilities. We will also try to identify other proteins that TANGO2 may interact with in healthy cells to perform its function(s).
Multi-Institutional Award: $50,000
RESEARCHERS

Joshua K. Meisner, MD
(PI)

Adam S. Helms, MD
(PI)
PROJECT
Platform for Screening Potential Treatments of TANGO2 Related Disease through TANGO2 Patient Derived Induced Pluripotent Stem Cell Cardiomyocytes and In Vivo Modeling
Abstract – TANGO2-related metabolic encephalopathy and arrhythmia (TRMEA) is a disorder caused by homozygous loss of function mutations in TANGO2, which is characterized by recurrent metabolic crises with severe cardiac consequences and neurologic symptoms. The clinical course of TRMEA after diagnosis is poor with a high frequency of sudden cardiac death and with no currently available treatments. Therapy development requires pre-clinical models for in vitro and in vivo testing on a clinically relevant phenotype, but there are no reported models to date that replicate the cardiac effects of TRMEA. Given the severe cardiac disease in patients, we will develop in vitro and in vivo models for testing potential therapies in two aims. In aim 1.1, we will first utilize cardiomyocytes differentiated from TRMEA patient induced pluripotent stem cells (iPSCs) and CRISPR-Cas9 edited knock out iPSCs on our novel human cardiac muscle bundle system to quantify the contractile and electrophysiologic dysfunction caused by loss of TANGO2 under metabolic stress. In aim 1.2-1.3, we will then use this system as high-fidelity testbed to assess rescue of cardiac defects with (1.3) AAV mediated gene therapy and (1.2) a focused set of pharmacologic therapies that have been proposed for use in TRMEA. Aim 2 will, for the first time, characterize cardiac defects in a TANGO2 knock out mouse model for subsequent translation of our results from aim 1 into an in vivo model. Together, these models will create the necessary bridge for translation of potential therapeutic targets in TRMEA to clinical care.
Lay Summary – TANGO2-related metabolic encephalopathy and arrhythmia (TRMEA) results in recurrent events often causing severe damage to the brain and heart. There is a critical need to develop treatments for this devastating disease that currently has no available treatments. Since TRMEA events have a severe effect on the heart, we will use our established model of heart muscle tissues “in a dish” to test the ability of gene therapy to rescue abnormal heart function with loss of TANGO2. These tissues additionally allow for testing of selected currently available drug therapies that have been proposed for use in TRMEA and the study will be combined with the first characterization of cardiac defects in a TANGO2 knock out mouse.
Single Institution Award: $25,000
RESEARCHERS

Na Li, PhD
(PI)

Lindsay Burrage, MD, PhD
(Co-PI)
PROJECT
Deciphering the mechanisms of TANGO2-deficiency induced cardiac arrhythmogenesis
Abstract – TANGO2-related metabolic encephalopathy and arrhythmia (TRMEA) is a recently described autosomal recessive disorder associated with biallelic TANGO2 variants. Individuals with TRMEA have a wide range of cardiac phenotypes including cardiac arrhythmias and cardiomyopathy, which may be life-threatening. The molecular mechanisms underlying the development of cardiac arrhythmias in the context of TANGO2-dysfcuntion remains elusive. The goal of this project is to understand the function of TANGO2 in cardiomyocytes and elucidate the molecular underpinnings of arrhythmia development in the Tango2 knockout mouse model. We will use a multidisciplinary approach to test the overall hypothesis that TANGO2-deficiency directly plays a causal role in the cardiac arrhythmogenesis. We will focus on the role of altered protein trafficking in the ion channel dysfunction due to the TANGO2-deficiency. The outcome of this study will provide a rationale to develop more effective therapeutics targeting the root cause of arrhythmia development in individuals with TRMEA.
Lay Summary – TANGO2-related arrhythmia is a rare genetic disorder caused by mutations in the TANGO2 gene. Affected individuals experience irregularities in the rhythm of the heart (arrhythmias), often triggered by fasting. Current treatment is aimed at the specific symptoms present in affected individuals. To develop a better treatment requires the understanding of the causal mechanisms that lead to the increased susceptibility to arrhythmia in the context of TANGO2 deficiency. Our research will utilize a mouse model of TANGO2-deficiency and investigate the molecular and cellular events that promote the arrhythmia development in the animal model, which could shed light on the optimal target for preventing and treating the arrhythmias in individuals with TANGO2 deficiency.
Single Institution Award: $25,000
RESEARCHERS

Lilei Zhang, PhD
(Co-PI – BCM)

Christina Miyake, MD MS
(Co-PI BCM)
PROJECT
Determine the molecular basis and the metabolic predisposition to TANGO2 crises using human induced pluripotent stem cell differentiated cardiomyocytes
Children with TANGO2 disease have multi system diseases, however, the heart disease is the major cause of death. The heart appears healthy at baseline, yet during crises electrical abnormality (arrhythmia) occurs and it is often resistant to standard therapy, which can cause lethality. Thus, understanding the mechanistic nature of these episodes, can help us design treatment strategies to minimize the chance of them happening and terminating these crises when they do occur.
With the support from the TANGO2 foundation, we have generated a single iPSC-CM (induced pluripotent stem cell differentiated cardiomyocytes) cell line from a patient with the common exon 3-9 deletion. These beating heart cells recapitulated the electrical abnormalities of TANGO2 patients in crises and importantly, we found the electrical abnormalities can be fully corrected by re-expressing normal TANGO2 in these cells. We believe this is the first cardiac model that faithfully recapitulate the condition of the TANGO2 patients and holds great potential to decipher disease mechanism and test promising treatment.
In the current proposal, we will generate isogenic (TANGO2 mutation corrected) controls of 3 additional patient iPSC lines carrying different mutations in TANGO2 gene. Then we will differentiate these iPSC (patient cells and their matching controls) into CM and investigate channel activities and trafficking as well as possible metabolic triggers for arrhythmia.
At the completion of the current proposal, we hope to confirm the molecular etiology of arrhythmia is due to trafficking defect of channels and identify potential metabolic stressors that induces arrhythmia as well as potential treatment strategies.
Lay Summary – We created human heart cells from one TANGO2 patient and showed that these beating heart cells in the dish is an effective cellular model for the heart disease in human patients with TANGO2 disease. In this proposal, we will make 3 additional TANGO2 patients and their matching control cells into heart cells and expand on the findings we had from the first patient. This allows us to validate the results in different TANGO2 cells and apply our work to all TANGO2 patients in the future. We will determine the reason of irregular heart rhythm during crises. By doing this, we will gain insights on both how to terminate these crises and how to avoid them. Our platform also allows us to test treatment including drugs and dietary supplements. The molecular mechanism we discover in the heart cells may also provide useful information for other organ systems. The studies proposed here will position us well for larger fundings from the NIH for more in-depth mechanistic studies and potential clinical trials in the near future.
Single Institution Award: $148,000
RESEARCHERS

Felix Distelmaier
MD (Co-PI)

Michael Sacher
PhD (Co-PI)
PROJECT
Characterization Of Human TANGO2: From Biological Function To Therapeutic Strategies
All human cells have a number of different compartments that include the nucleus, the endoplasmic reticulum and mitochondria to name a few. Each of these compartments has a unique set of proteins that give the compartment specific functions. Yet, compartments receive material from other areas of the cell in a process referred to as membrane traffic. Our research programs have focused on the consequences of mutations in human genes that affect mitochondrial function and membrane traffic. A prerequisite to devising a treatment plan for individuals with specific gene mutations is knowing what the affected protein does in a cell. In our current TANGO2 research project we focus on the cell physiological consequences of human TANGO2 deficiency. Our experimental approaches include live-cell-imaging, biochemical assays, metabolite profiling, proteomic analysis, and a large-scale chemical screening platform for drug discovery. One of our main interests is the elucidation of the cellular location of TANGO2 and the factors that influence this location. Moreover, we aim to gain more insights into the influence of TANGO2 on cellular energy metabolism as well as on membrane trafficking pathways, particularly between the endoplasmic reticulum and the Golgi. In this context we also hope to identify interaction partners of the TANGO2 protein, which would be of great help in understanding the biological function of TANGO2. Finally, we will apply a drug screening platform to search for chemical compounds that influence TANGO2 function and are able to mitigate the effects of TANGO2 deficiency.
Multi-Institution Award: $50,000
RESEARCHERS

Lina Ghaloul-Gonzalez, MD
(Co-PI)

Jerry Vockley, MD, PhD
(Co-PI)
PROJECT
Investigating the main function of TANGO2 and its role in mitochondrial dysfunction
TANGO2-related disorder is a genetic disorder caused by changes that inactivate both copies of the TANGO2 gene. While these changes have been identified in affected children, how they cause the characteristic symptoms of this disorder is unknown. Importantly, even patients who have the exact same mutation can have symptoms that vary from mild to severe for reasons that are unknown. TANGO2 is speculated to transport newly produced proteins in the cell to their final functional location. However, studies of this process have been limited. In preliminary experiments, I have shown that the ability of TANGO2 deficient cells (cells lacking TANGO2 protein from patients) to generate energy from mitochondria (the energy producing part of the cell) is impaired. I speculate that this defect arises from impaired transport of proteins necessary for normal mitochondrial function. It is important that once proteins are made, they are directed to the correct location in cells. My experiments will examine the normal location of TANGO2 protein in cells, and how that location is affected by mutations in TANGO2. Further studies will characterize energy production caused by TANGO2 mutations. In sum, these experiments will help us better understand the consequences of TANGO2 deficiency on the body and develop novel treatments for the disease.
Single-Institution Award: $25,000
RESEARCHERS

Christina Miyake, MD MS
(Co-PI BCM)

Lilei Zhang, PhD
(Co-PI – BCM)

Diana Milewicz, MD PhD
(Collaborator – UT Health Sciences)

Heinrich Taegtmeyer, MD PhD
(Collaborator -UT Health Sciences)
PROJECT
Creation of TANGO2 Patient-Derived Induced Pluripotent Stem Cells and Induced Pluripotent Stem Cell Differentiated Cardiac Myocytes to Gain Insights into the Molecular Basis of Cardiac Arrhythmia and Cardiomyopathy in TANGO2 Disease.
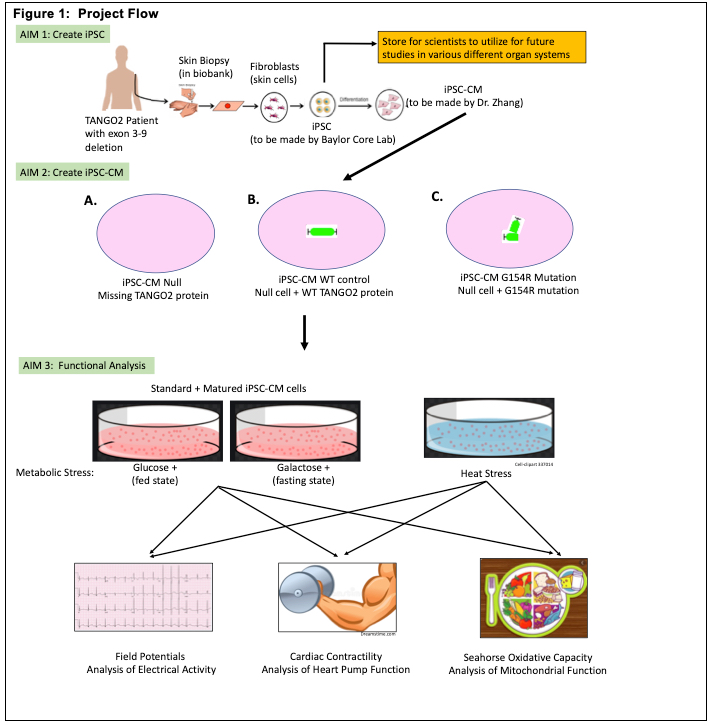
TANGO2 families have helped contribute to the natural history study and based on this data we have determined that metabolic crisis are triggered predominantly by illness, decreased oral intake, and heat. While the symptoms of TANGO2 disease are similar to mitochondrial and fatty acid oxidation disorders, the mechanism and role of TANGO2 remains unknown. We believe that understanding the mechanism behind TANGO2 may be best studied by looking at the heart because the heart is affected in all children during crisis and because it demonstrates the most severe effects. We believe that TANGO2 may play an important role in maintaining energy for cardiac cells. Stressors such as fasting or heat affect protein function in the heart. Ion channels are critical for the heart to beat normally and they maintain electrolyte balance in the heart cells. When alterations occur, this can lead to QTc prolongation, a hallmark of TANGO2 crisis, and heart failure, another new finding we are beginning to see.
Our study has 3 aims. First we will create induced pluripotent stem cell (iPSC) line that will be used for this study but can be used in future studies looking at all different organ systems. Second we will use the iPSC cells to create human heart cells. And lastly we will take these heart cells and put these cells under different stressors that are known to trigger crisis in TANGO2 children (example: we will “fast” the cells, denying the cells sugar and we will place the cells under heat). We will then study the effects that these stressors produce. We will specifically look to see how these stressors affect the cells metabolism, the ECG and heart function using specialized equipment in the Zhang lab. Then we will try and “rescue” the cells by adding certain nutrients and vitamins to see if we can reverse the effects.
The first aim of this study which uses fibroblasts from a TANGO2 child to create induced pluripotent stem cells will not only provide the foundation for us to study heart cells in this study but this will also provide the ability for other researchers in the future to study the different organs affected by TANGO2. This iPSC cell is a specialized cell that is the precursor cell for all cells within the body. By using an actual TANGO2 child’s cells, we will essentially be recreating the cells from that child’s body with all of their specific genetic information. These cells therefore, when studied, will provide insights into that specific child but the information can be generalized to other children with the same genetic change.
Single Institution Award: $28,000
RESEARCHERS

Pascale de Lonlay, M.D., PhD

Edor Kabashi, PhD
PROJECT
Deciphering the physiopathology of TANGO2 disease in human myoblasts and a novel zebrafish model
Rhabdomyolysis (RM) is an acute injury of skeletal muscle that results from environmental or congenital causes. TANGO2 is a recently discovered gene, whose mutations have been identified as a major cause of RM associated with encephalopathy. At the cellular level, TANGO2 protein presumably regulates the organization of the Golgi apparatus and the endoplasmic reticulum (ER), as well as mitochondrial function. In two different, but related monogenic disease leading to RM flares, our team has found that muscle cells exhibiting abnormal ER functions lead to an excessive inflammatory cascade. This can be caused partially due to defective lipid membranes. Given our findings in those two diseases, and state of the art in TANGO2 disease, we propose that perturbed vesicular ER/Golgi transport and/or mitochondrial functions from TANGO2 mutations contribute to the symptoms.
In order to follow our hypothesis, and to be able to find a potential treatment, we need good cellular and animal models that complement each other to study the disease. Zebrafish has emerged as an excellent model to study neurological, cardiac, and metabolic diseases that appear in humans. Our team has large experience on the study of the pathophysiology of neurological diseases such as ALS in zebrafish. Furthermore, zebrafish larvae serve as a great platform to screen medicines in a multi-well format, such that several medicines can be analyzed at the same time. Thus, we envision to create a mutant TANGO2 zebrafish using genetic engineering techniques (transient morpholino-mediated knockdown and stable CRISPR/Cas9 lines), in order to have a full organism model where we can, at the same time, do molecular investigations and screen medicines.
Taken together, combining molecular studies in primary human muscular cells and evaluating physiological parameters in TANGO2 mutant zebrafish, we expect to set the basis for the translation of potential drugs to higher vertebrates to protect patients from inevitably RM and neurological regression during febrile illness.
Multi-Institution Award: $50,000
RESEARCHERS

Claudia Soler-Alfonso, M.D., F.A.C.M.G.
PROJECT
Untargeted metabolomics in patients with TANGO2-related metabolic encephalopathy and arrhythmias.
TANGO2-related metabolic encephalopathy and arrhythmias is a severe medical condition presenting with muscle weakness, delayed developmental milestones, abnormal heart rhythms, and seizures. Patients can also present with life-threatening emergencies known as “metabolic crisis”. Individuals experiencing a metabolic crisis show low blood sugars, increased lactic acid, and increased ammonia levels in their blood. We do not understand the triggers and mechanisms behind the metabolic crisis. At our metabolic center, we observed strong similarities in patients with a TANGO2 metabolic crisis and metabolic crisis in patients unable to process fats in their diets due to specific genetic defects known as fatty acid oxidation disorders. However, standard biochemical tests in patients with TANGO2 do not show the same kinds of typical abnormalities seen in patients with fatty acid oxidation disorders. We do know, however, that TANGO2 patients have fewer episodes of metabolic decompensation when they are treated similarly to people with fatty acid oxidation disorders. Our research goal is to study TANGO2 patients using a technique called untargeted metabolomics to identify metabolic patterns in the blood that could give us an idea of why they experience episodes. This technique identifies hundreds of compounds instead of only a few dozen. It is a much more comprehensive way to look at chemicals in the blood. Our previous preliminary observations suggest that patients with TANGO2 have low levels of pantothenic acid (or vitamin B5), which is involved in fatty acid processing. Our research goal is to identify a specific metabolic profile in TANGO2 patients, with particular attention to vitamin levels and fat acid oxidation markers. If a definite pattern of deficiency is proven, we may be able to give patients extra amounts of a given vitamin, or other nutrients to make their symptoms better and prevent episodes altogether.
Single Institution Award: $25,000
RESEARCHERS

Mousumi Bose, PhD
Montclair University

Christina Miyake, MD, M.S.
Baylor College of Medicine
PROJECT
Determination of the Role of B-vitamin and Overall Nutrient Intake in Symptoms and Disease Severity in TANGO2 Deficiency Disorder
Abstract – Although anecdotal reports suggest B-vitamin supplementation may alleviate symptoms in TANGO2 deficiency disorder (TDD), no systematic study has evaluated the relationship between vitamin intake and disease severity. This pilot study will assess the role of B-vitamin and overall nutrient intake in the presentation and frequency of TDD symptoms. Using caregiver-reported 24-hour dietary recalls, we will quantify energy, macronutrient, and micronutrient intake in individuals with varying levels of disease severity. We will also collect symptom data using a standardized scale and examine correlations between nutrient intake and symptom presence and severity. This study will provide essential evidence for the potential therapeutic role of nutrition in TDD and inform future clinical trials and dietary recommendations.
Lay Summary – Many individuals with TANGO2 deficiency disorder (TDD) are given B-vitamin supplements, but there is currently no scientific consensus on whether these vitamins help or how much is needed. In this study, families of people living with TDD will work with a registered dietitian to report what their child eats and which supplements they take. The research team will also ask caregivers to report on common TDD symptoms, such as spells, seizures, and difficulties with movement or speech. By analyzing this information, the study will look for patterns between nutrition and symptom severity. The findings will help lay the groundwork for future research and could lead to clearer guidance on nutrition and supplement use for people with TDD.
Special Interest Award: $50,000
Award Year: 2025
RESEARCHERS

Christina Miyake, MD, MS, MPH (PI)
Baylor College of Medicine
PROJECT
TANGO2 Deficiency Disorder Natural History Study
Abstract: TANGO2 deficiency disorder (TDD) is a rare autosomal recessive genetic disorder characterized by severe metabolic and cardiac crises, impacting neurodevelopment and cardiovascular health. The disorder poses significant morbidity and mortality risks, particularly during episodes triggered by metabolic stressors.
This study aims to establish a robust biorepository and comprehensive clinical database of individuals with TANGO2 deficiency disorder to facilitate future scientific research and therapeutics. Specific objectives include:
- Biorepository Establishment:To collect and store biological samples, including blood and optional skin biopsies, from probands and their first-degree relatives diagnosed with TANGO2 deficiency disorder. These samples will serve as a critical resource for ongoing and future genetic and functional studies.
- Clinical Database Development:To compile detailed clinical data through comprehensive chart reviews and patient interviews. Diagnostic studies, including exome and genome sequencing, will be conducted to confirm TANGO2 mutations and explore genetic underpinnings.
- Patient Identification and Recruitment:Patients diagnosed with TANGO2 deficiency disorder will be identified through collaborative efforts with Texas Children’s Hospital, Baylor Genetics Laboratories, the TANGO2 Research Foundation, and relevant medical networks. Physicians and genetic counselors will facilitate patient enrollment, ensuring adherence to ethical guidelines and informed consent protocols.
- Functional and Molecular Studies:Biological samples collected will support functional studies aimed at understanding disease mechanisms and potential therapeutic targets. Transformed cell lines, induced pluripotent stem cells (iPSCs), and DNA will be made available to qualified investigators for molecular studies contributing to the broader understanding of TANGO2 disease pathophysiology.
Eligible participants will undergo thorough clinical evaluations, including electrocardiograms, echocardiograms, and other relevant diagnostic tests. Research protocols will be explained to participants and their families, and informed consent will be obtained prior to data and sample collection. Collaboration with the TANGO2 Research Foundation will facilitate outreach to affected families and support ongoing participant engagement.
The TANGO2 Natural History Study represents a pivotal effort to advance knowledge of TANGO2 deficiency disorder through comprehensive clinical and molecular investigations. By establishing a biorepository and clinical database, this study aims to catalyze future research initiatives, ultimately improving diagnostic accuracy, treatment strategies, and outcomes for individuals affected by TANGO2 deficiency disorder..
Lay Summary: The purpose of this study is to define the natural history of TDD. The objectives of this study are: 1. To establish a biorepository of individuals with TANGO2 deficiency disorder to support future scientific research and treatments. 2. To establish a comprehensive clinical database of individuals with TANGO2 deficiency disorder. 3. Identify patients suspected to have TANGO2deficiency disorder through Texas Children’s Hospital. This research is crucial in addressing the unmet needs of the TANGO2 community and underscores the importance of collaborative efforts in rare disease research.
Award: $85,000
Award Year: 2024
RESEARCHERS

Andrea Wilderman, PhD (PI)
Baylor College of Medicine

Lilei Zhang, MD, PhD (Project Mentor)
PROJECT
Characterization of TANGO2 Deficient Neurons Derived from Human Induced Pluripotent Stem Cells
This project is co-funded by the TANGO2 Research Foundation and Uplifting Athletes. Uplifting Athletes is a nonprofit organization that connects student-athletes across the country as well as professional athlete ambassadors with their local rare disease community to raise awareness and funds, and bring inspiration and hope to those impacted by rare diseases, which is approximately one in 10 Americans. The annual Young Investigators Draft is inspired by the NFL draft, invests in research which could lead to potential rare disease treatments and cures. Just as the NFL Draft rallies around the hope and possibilities that the next generation of game-changing talent will bring a franchise, the Young Investigators Draft celebrates the early-career research investigators who are working toward a brighter future for those affected by rare diseases.
Abstract – TANGO2 deficiency disorder (TDD) is a multisystem disease with neurodevelopmental effects including seizures, sudden hypotonia and loss of motor control. In the recent TANGO2 Research Foundation family conference, all families unanimously expressed their interest in understanding and improving the neurological symptoms of their affected children. Progress on understanding the neurological aspects of TDD is hampered by a lack of biologically relevant models. Our lab uses induced pluripotent stem cell (iPSC) models to study the electrophysiological characteristics of cardiac myocytes to understand the physiology underlying sudden cardiac crises seen in TDD patients. We have produced isogenic pairs of iPSCs where TANGO2 is knocked out (KO) or left intact (WT). Study of cardiomyocytes derived from these TANGO2-KO and WT iPSCs with the same genetic background has produced highly reproducible phenotypes. Using these same iPSC lines, we will overexpress specific transcription factors to produce WT and TANGO2-KO excitatory and inhibitory neurons. We will characterize neuronal network properties through measurement of electrical activity and the coordination of signal across the network. Additionally, we will generate transcriptomic profiles of both neuronal types. As this is the first human neuron model of TDD, we expect differences in gene expression between WT and TANGO2-KO neurons will reveal neuron-specific pathways associated with TANGO2 deficiency that can be used in the development of treatment strategies. These results will be used to inform further analysis of cellular and molecular phenotypes associated with TANGO2 deficiency and our ongoing behavioral studies using mouse models.
Lay Summary – TANGO2 deficiency disorder (TDD) affects multiple body systems, causing neurodevelopmental issues like seizures and loss of motor control. Families at the recent TANGO2 Research Foundation conference are keen to understand and improve neurological symptoms in their affected children. However, progress is slow due to a lack of suitable models. In our lab, we use induced pluripotent stem cell (iPSC) models to study cardiac cells and comprehend the physiology behind sudden cardiac crises in TDD patients. We’ve created iPSCs with and without TANGO2, observing reproducible traits. Next, we’ll use these iPSCs to generate neurons and explore their network properties and gene expression. This human neuron model of TDD aims to reveal pathways associated with TANGO2 deficiency for future treatments. These findings will guide further analysis of TANGO2 deficiency and ongoing studies with mouse models.
Award: $20,000 (Funded by TANGO2 Research Foundation & Uplifting Athletes)
Award Year: 2024
RESEARCHERS

Nishanthi Mathiyalagan, PhD (PI)
Brigham and Women’s Hospital/Harvard Medical School

Vandana Gupta, PhD (Project Mentor)
PROJECT
Dynamic Metabolic Changes in TANGO2 Deficiency Contributing to Disease Onset and Progression In Vivo
This project is co-funded by the TANGO2 Research Foundation and Uplifting Athletes. Uplifting Athletes is a nonprofit organization that connects student-athletes across the country as well as professional athlete ambassadors with their local rare disease community to raise awareness and funds, and bring inspiration and hope to those impacted by rare diseases, which is approximately one in 10 Americans. The annual Young Investigators Draft is inspired by the NFL draft, invests in research which could lead to potential rare disease treatments and cures. Just as the NFL Draft rallies around the hope and possibilities that the next generation of game-changing talent will bring a franchise, the Young Investigators Draft celebrates the early-career research investigators who are working toward a brighter future for those affected by rare diseases.
Abstract – Bi-allelic pathogenic mutations in TANGO2 cause TANGO2-deficiency disorder (TDD), a rare metabolic disorder associated with defective lipid metabolism. Our research focuses on identifying the in vivo triggers contributing to disease onset by studying the temporal changes in the cellular lipidomic of tango2-deficient zebrafish from pre-symptomatic to disease onset and finally during the chronic disease state. Based on these findings and the current knowledge of genes involved in lipid metabolism, we will create new zebrafish models by overexpression or knockdown studies. Characterization of these zebrafish models will shed light on the role of these genes in lipid metabolism and their interactions with TANGO2. This will enable us to identify a possible causal pathway that forms the basis of TDD treatment strategies.
Lay Summary – TANGO2-deficiency disorder, a rare metabolic disease, has been found to be associated with defective lipid metabolism. Our research focuses on evaluating the lipid profile of tango2-deficient zebrafish at various developmental time points to understand the fatty acid pathways that may be perturbed, contributing to the disease condition. Findings from this study will help us develop viable options for TDD treatment and management.
Award: $20,000 (Funded by TANGO2 Research Foundation & Uplifting Athletes)
Award Year: 2023

